Description
B. Braun Deep Access Introcan Safety® IV Catheter, 24mL/min, 22GX2.5,50 each/box, 4251622-02
Latex content: Latex-Free
Brand: Safety®
Introcan Safety® Deep Access IV Catheters, 22GA, 2.5in.
Longer Length Peripheral Catheters help reduce the risk of catheter failure and complications associated with accessing deeper veins
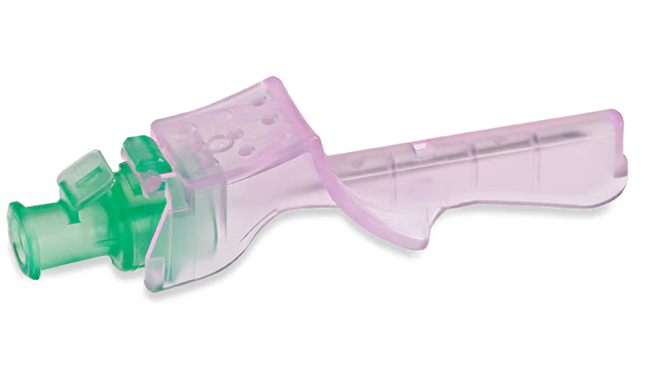
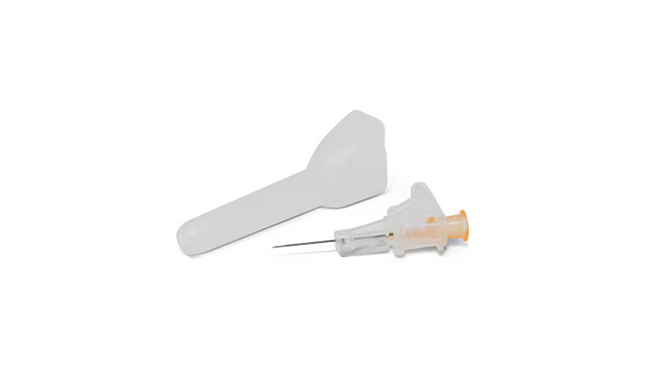
22Ga Introcan Safety® Deep Access
The Introcan Safety® Deep Access IV Catheters are designed to facilitate ultrasound-guided procedures where access to deeper veins is desired. These types of IV procedures are usually required for patients with difficult vascular access, including overweight/obese patients. Short PIVCs have been associated with several complications related to deeper vessels, such as catheter-related thrombophlebitis, dislodgment, and infiltration. The Introcan Safety Deep Access IV Catheters are designed to allow clinicians to leave more catheter in the vessel and help reduce the risk of complications.
The Introcan Safety Deep Access IV Catheters are part of the Introcan Safety family of IV catheters and include all the same features that have made Introcan Safety a leader in peripheral vascular access.
Introcan Safety Deep Access IV Catheters are:
Visible under ultrasound. The needle tip and catheter are visible under ultrasound, providing a visualization aid during the insertion process.5
Made using polyurethane material. Polyurethane and longer lengths associated with extending the duration of therapy.1
Designed to prevent accidental needle sticks. The fully automatic passive safety shield requires no manual activation, CANNOT BE BYPASSED, and prevents needle reinsertions.
Product Information & Image Disclaimer
We strive to provide accurate product details and images; however, product specifications, ingredients, packaging, and appearance may change without notice. Images may not always reflect the actual product. For the most current and accurate information, please refer to the product packaging and the manufacturer’s official website.